April 12 2016
FlashLink Certified Vaccine Data Logger with Glycol Bottle Now Recommended for Purchase by UN Agencies
DeltaTrak®, a leading innovator of cold chain management solutions today announced its FlashLink Certified Vaccine Data Logger with Glycol Bottle has been granted prequalification status by the World Health Organization (WHO). The prequalification grant validates that the logger has been shown to comply with the relevant WHO PQS performance specifications and has been independently assessed against WHO verification protocols.
“We are very proud to be granted this approval and recognition that our logger meets the WHO guidelines,” said Frederick Wu, president and CEO of DeltaTrak. “This is the very first data logger with a glycol bottle to be prequalified by the WHO enabling UN agencies, governments and other organizations access to an automated method of monitoring temperature sensitive vaccines stored without being affected by fluctuations in air temperatures caused by unit cycling or frequent opening and closing of storage doors.”

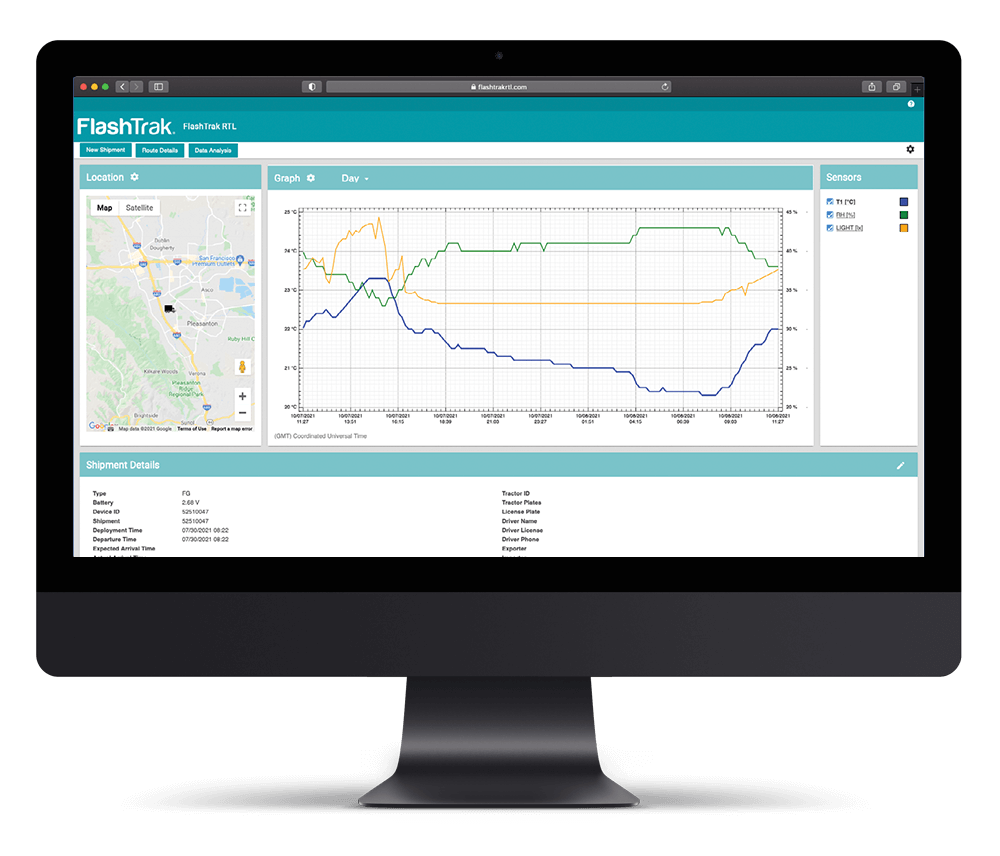
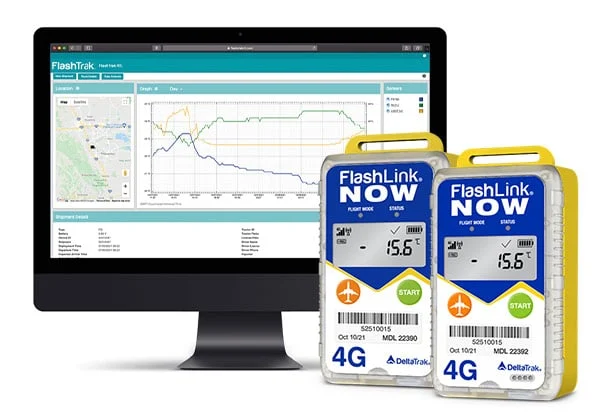
The FlashLink Certified Vaccine Data Logger with Glycol Bottle is user-programmable and has a temperature sensor enclosed in a bottle of propylene glycol. It is designed for monitoring temperature conditions of vaccine storage units in clinics, hospitals, and other vaccination storage facilities. The glycol bottle provides a buffer against temperature fluctuations expected during normal use, such as frequent door openings and cycling of the refrigeration/freezer unit. Each logger is individually certified and comes with an NIST traceable Certificate of Calibration. The LCD allows users to see current temperature, scroll through statistical data (high, low, average temperature, time in alarm), and view battery life status. A flashing red LED visually alerts when temperature excursions occur. The logger stores up to 16,112 data points in a non-volatile memory, and downloads to a PC for record keeping and data analysis using FlashLink Program Manager Software.
About DeltaTrak®
DeltaTrak® is a leading innovator of cold chain management, environment monitoring and food safety solutions for the food, pharmaceutical, life sciences and chemical industries. Contact DeltaTrak by phone at 1-800-962-6776 or by email at [email protected]. Additional information can be found at www.southpacific.deltatrak.com.