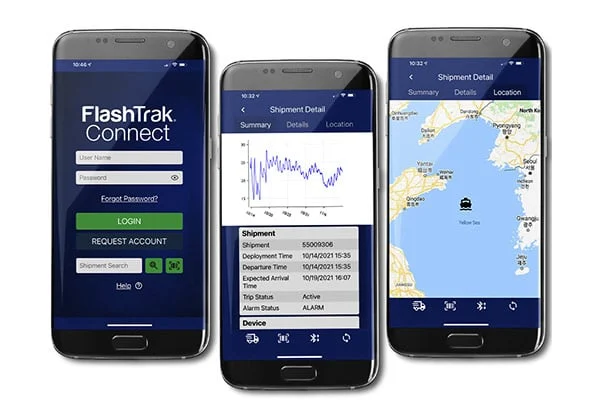
Compromised shipments
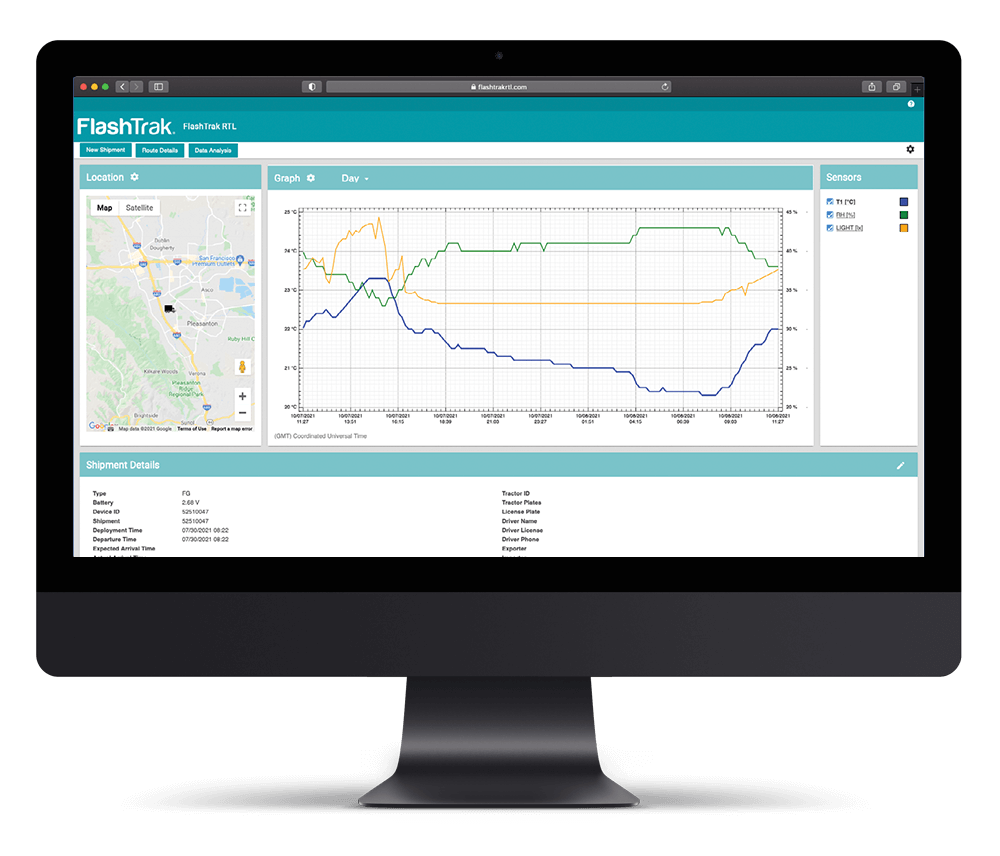
Optimum conditions need to be maintained for drugs designated for clinical trials across the supply chain. Ineffective temperature monitoring can impact the whole process.
Human error
Efficient temperature monitoring is crucial before clinical trials begin, but too often human error can lead to mistakes in record keeping or even failure to activate data loggers.

Continuous monitoring
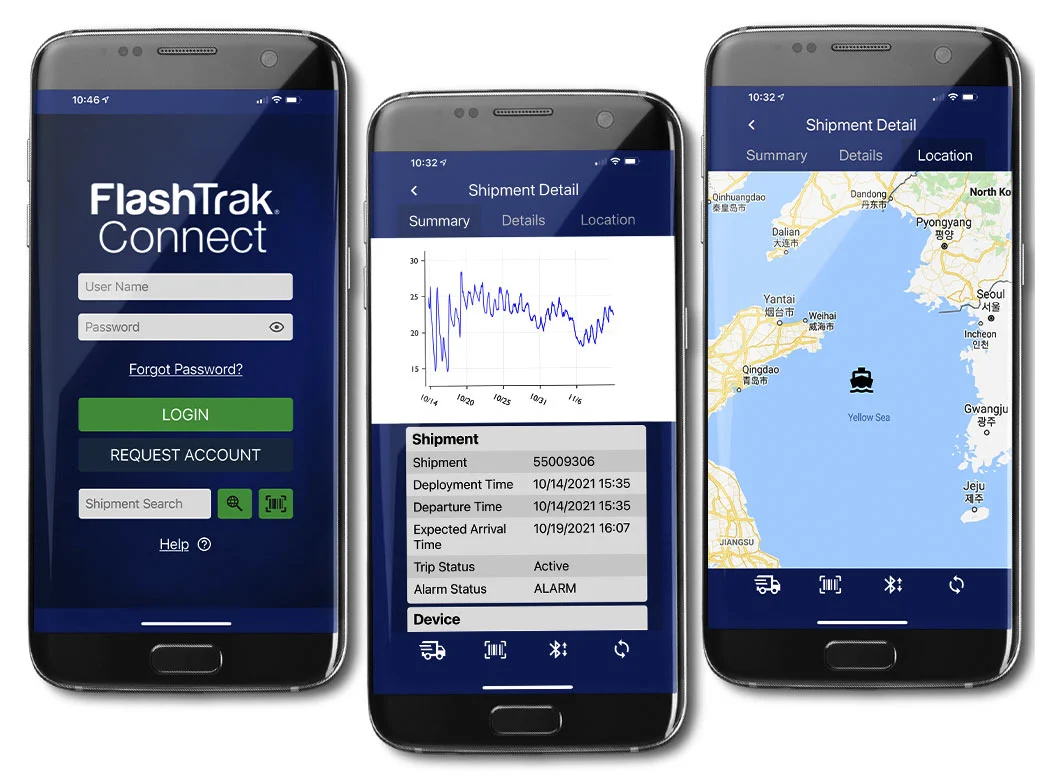
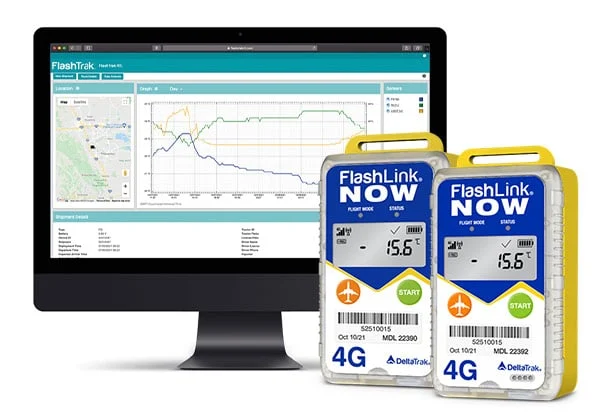
DeltaTrak’s solutions deliver cost-effective, continual monitoring throughout the cold chain and storage stages, delivering accurate records even if the logger is not started.